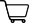On May 5th 2013, results from the AREDS2 clinical trial were presented at the ARVO annual meeting and published online in the Journal of the American Medical Association (JAMA).
In the first AREDS trial, supplementation with vitamins C and E, zinc, copper and beta-carotene reduced risk of AMD progression by 25%. In AREDS2, the investigators found no overall additional benefit from adding omega-3 fatty acids and/or lutein and zeaxanthin to the original formula. Importantly, however, an 18% reduced risk of developing advanced AMD was seen for those who received a beta-carotene free version of the AREDS formula plus lutein (10 mg) and zeaxanthin (2 mg).
An even greater, 26% reduction in risk for progression to advanced AMD was seen for those with low dietary intake of lutein and zeaxanthin (median 0.7 mg per day), who took an AREDS formula plus lutein and zeaxanthin compared to those with similar, low dietary intake who did not receive lutein and zeaxanthin. That finding is of striking public health importance since a substantial portion of Americans consume very little of these carotenoids.
Below, Dr. Emily Chew summarized the the nutritional formula now recommended for those with intermediate to advanced age-related macular degeneration, based on the AREDS 2 findings:
Red lines indicate nutrients to exclude; gold text indicates a new formula addition:

Dr. Emily Chew, presenting the AREDS2 findings at ARVO, pointed out that study participants were very well-nourished, and suggested that a greater reduction in AMD progression may have been demonstrated if the subjects’ diets had been more representative of the general U.S. population. Dietary intake of lutein and zeaxanthin for Americans is typically 1-2 mg per day. Additionally, Dr. Chew emphasized that beta-carotene was observed to suppress serum levels of lutein when these carotenoids were co-supplemented, due to their competitive absorption.
While no increased lung cancer risk was seen in those taking lutein and zeaxanthin, there was an association between beta-carotene and risk of lung cancer among former smokers. About half of AREDS2 participants were former smokers. Thus, the National Eye Institute now recommends adding lutein and zeaxanthin to the original formula and eliminating beta-carotene as a safer formulation for those with moderate to advanced AMD.
The trial also compared the effects of a lower dose of zinc (25 mg) with the original 80 mg administered in AREDS. A trend was seen that favored the high dose zinc for better protection against progression, though it did not reach significance. The authors conclude that there is insufficient data to make a new recommendation for zinc, and the NEI recommends that those with moderate to severe AMD continue to use the 80 mg dose. Importantly, no clinically or statistically significant differences in adverse effects (exacerbations in some GI and genitourinary conditions) were seen in the high versus low dose zinc groups.
Dr. Chew emphasized that the benefits of taking the original AREDS formula (with lutein and zeaxanthin, but no beta-carotene) apply to both the geographic atrophy and neovascular forms of AMD in those with moderate to advanced disease. Benefits of taking AREDS supplements are also long-lasting, according to Dr. Chew. An April, 2013 follow-up report in the journal Ophthalmology found that participants of the first AREDS trial taking the original antioxidant and zinc supplements, had a 25-30% reduced risk of developing advanced AMD over the next 5 years.
Looking Forward:

ScienceBased Health’s MacularProtect Complete® AREDS2 reflects the NEI’s new recommendations, based on the AREDS 2 findings, in its levels of vitamins C, E, zinc, copper, lutein – and exclusion of beta-carotene. It includes 1 mg of zeaxanthin, which will be increased to the 2 mg tested in AREDS2 and available shortly. MacularProtect Complete AREDS2 includes a comprehensive multinutrient for whole body health. MacularProtect Complete AREDS2 is also available as a drink mix. MacularProtect® AREDS2, a stand-alone AREDS formulation, also reflects the AREDS 2 findings. For more information on SBH macular products, visit www.sbh.com or contact us for more information: CustomerCare@SBH.com or 888-433-4726.
In a separate study, published online in JAMA Ophthalmology, AREDS2 also evaluated the effect of the various AREDS formulas on cataract. While none of the formulations helped reduce the risk of progression to cataract surgery, a subgroup of participants with low dietary lutein and zeaxanthin did gain some protection. In those with the poorest intake of lutein and zeaxanthin, there was a 32% reduction in progression to cataract surgery, a 30% reduction in development of any cataract, and a 36% reduction in the development of any severe cataract.
View a more in-depth discussion of the AREDS2 trial results in the May, 2013 SBH EduFacts newsletter